Gastromotal®
¹³C-octanoic acid breath test
¹³C-octanoic acid breath test used for in vivo diagnosis of solid gastric emptying rate and allows to detect gastric motor disorders. The ¹³C-octanoic acid breath test represents a novel method for the assessment of gastric emptying of solid nutrients and compared with the current standard methods (i.e. scintigraphy).
The test is made available for clinical research, and INFAI welcomes inquiries from researchers and physicians interested in using the test.
Features
Non radioactive
Scintigraphy uses radioactive isotopes. ¹³C is a stable isotope and therefore inherently safer than a test with radioisotopes.
Cost-effective
The ¹³C-octanoic acid breath test can be performed at lower costs compared to the cost of scintigraphy and is more accurate than ultrasound imaging.
Present status of Gastromotal
Gastromotal is accepted as eligible for a faster centralized authorization procedure by the European Medicines Agency (EMA). Gastromotal was submitted for review by EMA in 2007. Application was reviewed positively, but since EMA requires an additional clinical trial, application was put on hold until a therapy is available which Gastromotal test could accompany. INFAI plans a clinical study with a new medicinal product (Relamorelin) showing a high efficiency for treatment of diabetic gastroparesis, thus now being able to provide the clinical results requested by EMA for market approval.
The faster procedure for approval, with which we have considerable experience, will enable to enter the market within one year after concluding the clinical trial lasting for twelve months, therefore in 2024. Gastromotal on the EMA website
Indication for Gastromotal
¹³C-octanoic acid breath test used for in vivo diagnosis of solid gastric emptying rate and allows to detect gastric motor disorders. The ¹³C-octanoic acid breath test represents a novel method for the assessment of gastric emptying of solid nutrients and compared with the current standard methods (i.e. scintigraphy).
Stomach motility is affected by a number of clinical conditions, including diabetes, non-ulcer dyspepsia, GERD and some post-operative conditions. Recent developments of drugs that affect gastric motility have led to renewed interest in this topic. Gastromotal on the EMA website
Test principle and protocol of Gastromotal
The techniques currently used to determine gastric emptying (scintigraphy, MR, blood test and ultrasound) have significant shortcomings, so a new, better test will be of significant value. Ghoos et al (Gastroenterology, Vol 104, 1640-1647, 1993) described a ¹³C-octanoic acid breath test in which the substrate is presented with egg in a test meal and the emergence of ¹³CO₂ in breath is monitored. ¹³C-octanoic acid is a normal component of foods such as butter, so no negative side effects are expected. The mathematical analysis of the excretion curve, using a method developed by INFAI, allows to determine the gastric emptying rate. The test allows non-invasive detection of patients with gastric motility problems and evaluation of motility enhancing drugs.
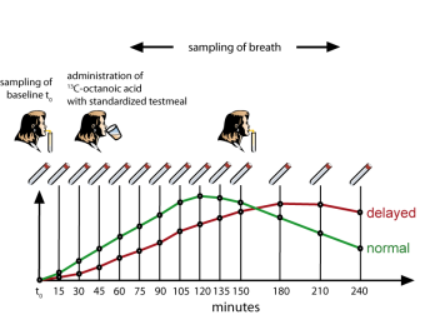
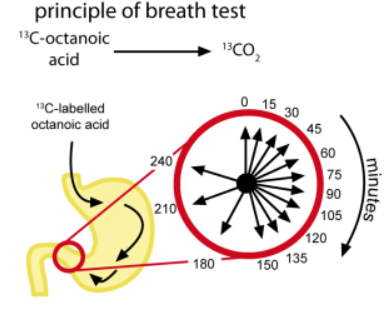
The patient consumes ¹³C-octanoic acid on an empty stomach. It is mixed with raw egg yolk, fried and served with bread, butter and 150 ml coffee or tea. In this form, the ¹³C-octanoic acid is only released upon arrival in the small intestine, where it is absorbed and quickly metabolized in the liver. The resulting ¹³CO₂ appears almost immediately in the breath and is collected in glass sample tubes into which the patient breathes with a straw, which are sealed and stored until analysis. Two breath samples are taken directly before the test, ten samples at 15-minute intervals after the test meal, and another three at half-hourly intervals, covering a total measurement time of 240 minutes. The tubes are sent to a qualified laboratory for ¹³CO₂ analysis and the excretion curve is mathematically analyzed to distinguish between normal and impaired gastric motility. Two parameters (gastric emptying coefficient and half time of gastric emptying) are calculated and reported using a mathematical procedure and software developed by INFAI.
More Information
Ongoing studies for Gastromotal
Clinical studies for Gastromotal
Study number: OA 99/1/001
Validation of the 13C-octanoic acid breath test for the determination of gastric emptying rate in comparison to radioscintigraphy with 99mTc-albumin colloid. Sponsor: INFAI
Study number: AA00/01
Open, controlled, two centre, prospective, reference method controlled study investigating the diagnostic properties of the 13C-octanoic acid breath test in comparison to the gold standard, radioscintigraphy with 99mTc-albumin colloid. Sponsor: INFAI
Clin. Report No: S246.1.102.01
Single, oral, rising-dose, placebo controlled, double-blind study ofKC 11458 to investigate safety, tolerability and pharmacokinetics in healthy male volunteers. Sponsor: Solvay Pharmaceuticals
Clin.Report No: S246.1.104.01
Double-blind, randomized, placebo-controlled, single-dose, 4-way crossover study of 3 doses of KC 11458 to investigate the effect on gastric emptying in diabetic patients suffering from delayed gastric emptying. Sponsor: Solvay Pharmaceuticals
Clin. Report No: S246.1.109.01
Intravenous, placebo-controlled, repeated rising-dose, randomized, double-blind study of KC 11458 to investigate safety, tolerability and pharmacokinetics in healthy male volunteers. Sponsor: Solvay Pharmaceuticals
Clin. Report No: S246.1.110.01
Open, randomized, baseline placebo-controlled, 4-way eross-over study of single oral doses of KC 11458 to investigate. the influence of enteric-coating with respect to a corresponding meal on the pharmacodynamics and pharmacokinetics of KC 11458 in healthy male volunteers. Sponsor: Solvay Pharmaceuticals
Clin. Report No: S246.1.107.01
Single, intravenous, rising-dose, placebo-controlled, double-blind study of KC 11458 to investigate safety, tolerability and pharmacokinetics in healthy male volunteers. Sponsor: Solvay Pharmaceuticals
Ongoing studies for Gastromotal
Study: Gastromotal; Eudra CT Number: 2011-002782-38
Gastromotal 1-¹³C-Caprylic Acid breath test in the diagnosis and evaluation of therapeutic outcome in patients with dyspeptic symptoms and delayed emptying. Sponsor: INFAI
Gastromotal Patente
- EP1553987, Method for determining gastric evacuation using a 13C-labelled test meal, European Patent, 01.08.2007
- US20060057181, Method for determining gastric evacuation using a 13C-labelled test meal, US Patent, 16.03.2006
- US20120071728, Method for determining gastric emptying, US Patent, 22.03.2012
- WO2004037298, Method for determining gastric evacuation using a 13C-labelled test meal, International Patent, 06.05.2004
References
- Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G (1993): Measurement of gastric emptying rate of solids by means of a carbons-labeled octanoic acid breath test. Gastroenterology 104(6): 1640-7. PUBMED
- Pfaffenbach B, Wegener M, Adamek RJ, Wissuwa H, Schaffstein J, Aygen S, Hennemann O (1995): Non-invasive ¹³C octanoic acid breath test for measuring stomach emptying of a solid test meal–correlation with scintigraphy in diabetic patients and reproducibility in healthy probands. Z. Gastroenterol. 33(3): 141-5. PUBMED
- H. Steinbrede, S. Aygen, C. Steinborn (1997): KC 11458, a new motilin agonist, is effective in the acceleration of gastric emptying in healthy male volunteers Gut 29: A155 mit dem doi: https://doi.org/10.1016/S0016-5085(98)83430-6.
- Choi, MG., Camilleri M., Burton DD., Zinsmeister AR., Forstrom LA., Nair KS. (1998): Reproducibility and simplification of ¹³C-octanoic acid breath test for gastric emptying of solids. Am. J. Gastroenterol. 93: 92-98. PUBMED
- Maes BD., Spitz B., Ghoos YF., Hiele MI., Evenepoel P, Rutgeerts PJ. (1999): Gastric emptying in hyperemesis gravidarum and non-dyspeptic pregnancy. Aliment Pharmacol Ther 13: 237-243. DOI
- H. Steinbrede, S. Aygen, G.Eibes, C. Steinborn: The effect of multiple doses of KC 11458, a new motilin agonist, on gastric emptying of a solid test meal in healthy male volunteers, Gastroenterology 114, No. 4, G3455 DOI: https://doi.org/10.1016/S0016-5085(98)83431-8
- RUSSO, J. E. STEVENS, N. GILES, G. KRAUSE, D. G. O’DONOVAN, M. HOROWITZ, K. L. JONES: Effect of the motilin agonist KC 11458 on gastric emptying in diabetic gastroparesis, Aliment Pharmacol Ther 2004; 20: 333–338. DOI
